In part 1 of our look at different colloidal drug carrier systems used by pharmaceutical researchers, we highlighted nanosuspensions and lipsomes.
Today in part 2, we continue with a look at mixed micelles and colloidal liquid crystalline structures.
- Mixed Micelles
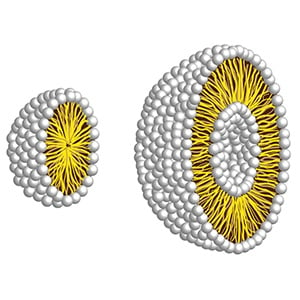
Mixed micelles are comprised of compounds that have poor water solubility, and are dissolved in the center and thus blend with the hydrophobic tails (these are two water repellent string-like objects attached to the hydophilic heads).
Mixed micelles are the primary way in which lipids (i.e. organic compounds that are fatty acids or derivatives of fatty acids that are insoluble in water, but soluble in organic solvents) transfer to the intestine’s cell surface, so that they can be absorbed.
Mixed micelles typically have high critical micelle concentration (CMC), and thus are unstable when strongly diluted (such as is the case when diluted in blood). Furthermore, some mixed micelles have toxic side effects, and they also exhibit an unpleasant taste in peroral liquids due to the tensides (detergents).
- Colloidal Liquid Crystalline Structures
There are two types of liquid crystalline phases (a.k.a. mesophases): lytropic and thermotropic.
Lytropic liquid crystalline phases are materials that produce liquid crystals by adding solvents, so that the concentration of water soluble amphiphiles (a special class of surface active molecules called surfactant) increases. This is necessary in order to prevent the solvents from dissolving them. Temperature also plays a key role in lytropic liquid crystalline phases.
Thermotropic liquid crystalline phases do not have a distinct melting point, but produce crystals within a certain temperature range. Pharmaceutical researchers leverage this outcome in their work by maintaining the liquid crystalline states of their drugs at lower (i.e. “supercooled”) temperatures.
Both lytropic and thermotropic liquid crystals are used as drug delivery systems because they can improve the dissolution of drugs that are otherwise poorly water soluble. However, as with mixed micelles, tenside concentrations are relatively high and the collodidal dispersions occur only within a very specific, slim range of parameters. And while they are thermodynamically stable and self-assembling, they revert to their prior basic micellar or molecular dispersed state when water is added.
Stay Tuned for Part 3
In part 3 of our overview of colloidal drug carrier systems, we will explore microemulsions and nanoemulsions.
Pion Technology: Trusted by Pharmaceutical Researchers
Our innovative technology is trusted by drug researchers and lab managers around the world because it delivers an array of key benefits for producing nano/micro emulsions and dispersions, lipids and suspensions for a variety of applications, including: injectables, vaccines, targeted drug delivery, inhalants, time release, anesthetics and antibiotics.
In addition, we have extensive experience in the challenges that our pharmaceutical customers face as they transition from concept, through to R&D, clinical trials, all-important FDA approval and finally, to manufacturing.
Learn more here.

